Human Plasma Derived Products
Akron’s proven blood products deliver safety and performance backed by robust and redundant supply. Because you can’t afford to compromise.
Our human blood-derived products are engineered specifically with our advanced therapy customer’s needs in mind. We've designed each of these products to deliver optimal cell growth and viability within cGMP manufacturing processes, never compromising on safety and lot-to-lot consistency.
These products are backed
with comprehensive manufacturing and pathogen safety documentation that aims to
meet commercial regulatory requirements of leading health authorities within
the US, EU, and Asia.
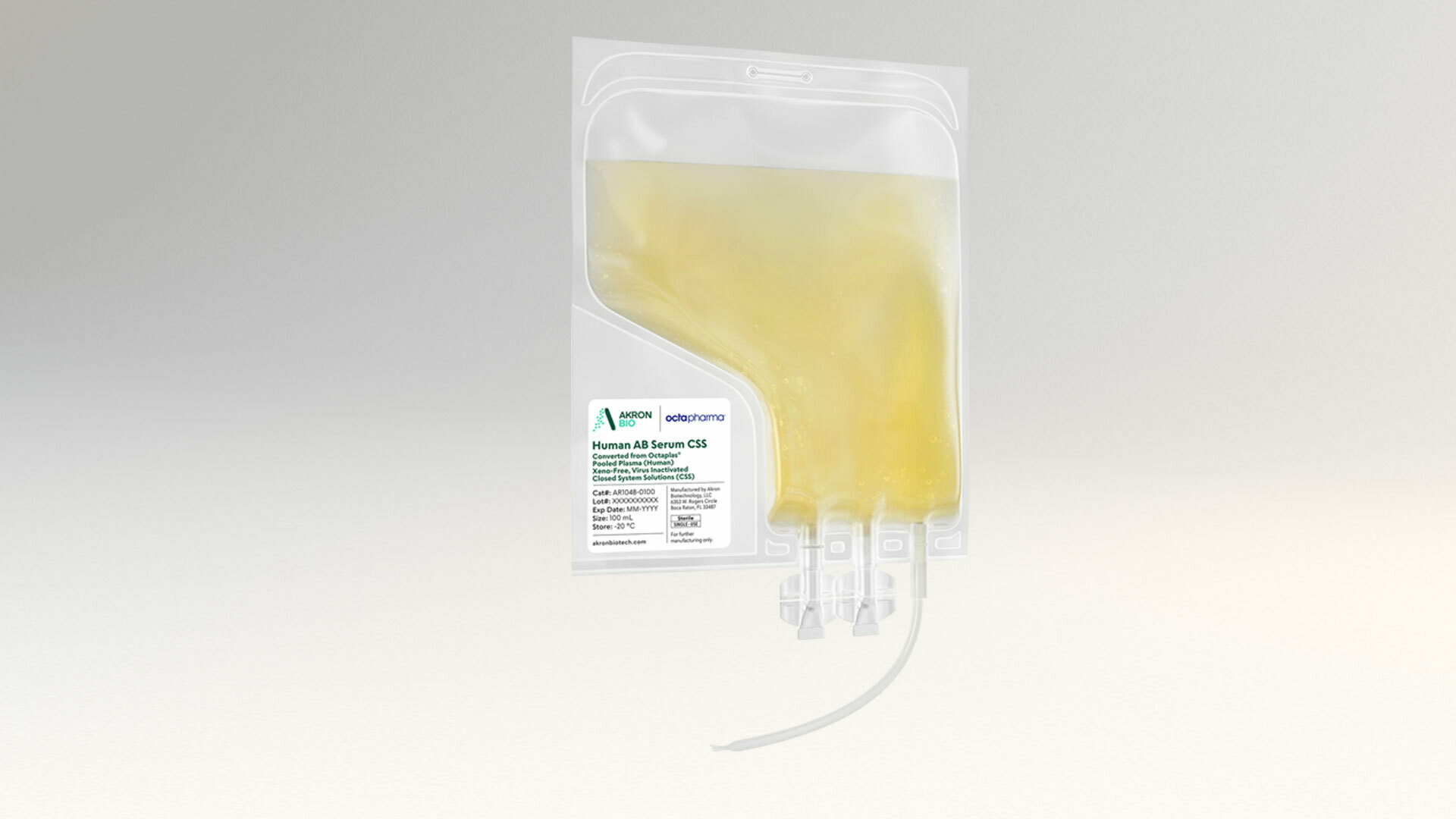
Human AB Serum CSS, Converted from Octaplas®, Pooled Plasma (Human), Xeno-Free, Virus Inactivated

Human AB Serum, Converted from Octaplas®, Xeno-Free, Virus Inactivated
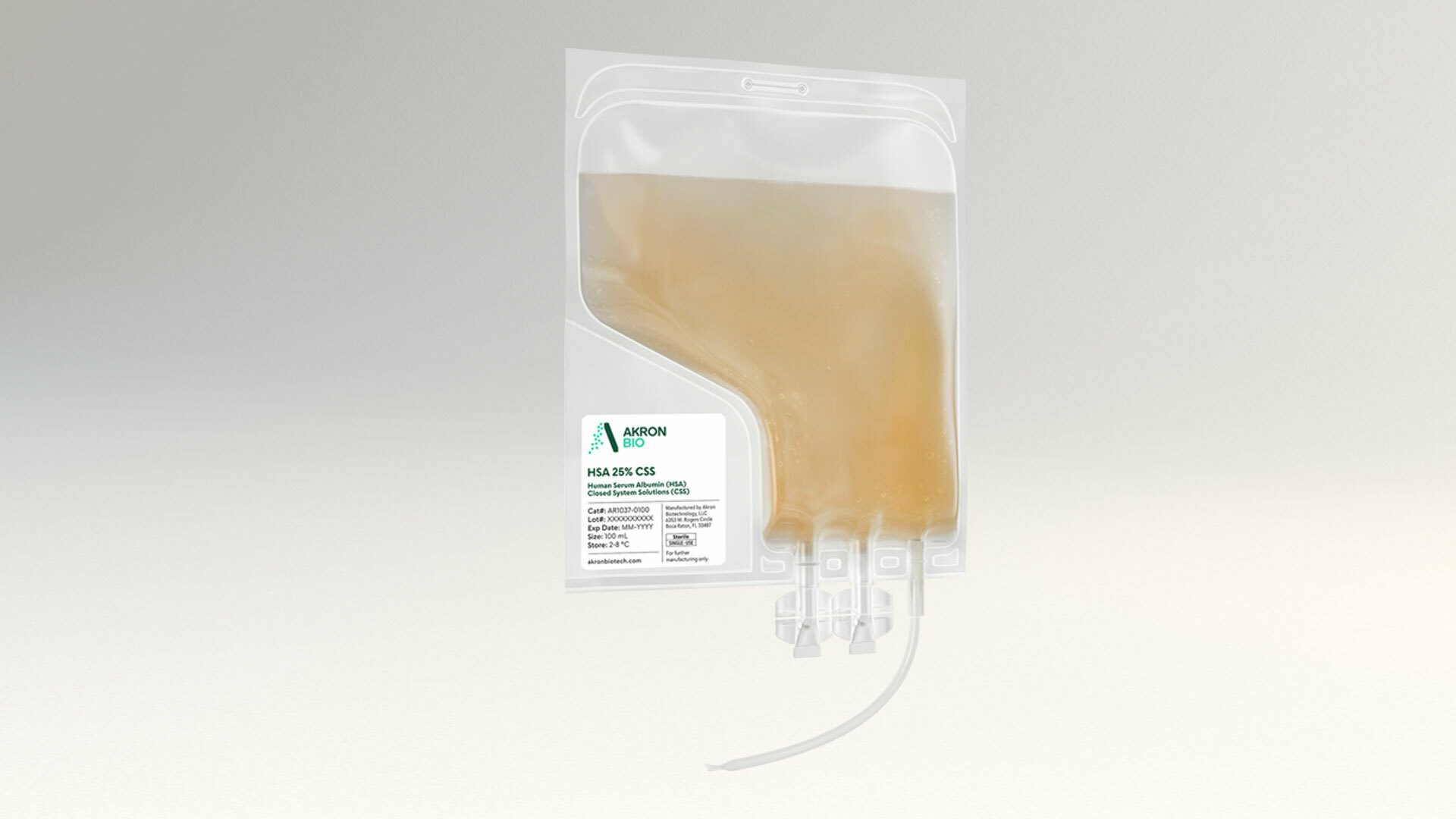
Human Serum Albumin 25% Solution CSS

Human Serum Albumin 25% Solution

Human Fibronectin, Virus Inactivated
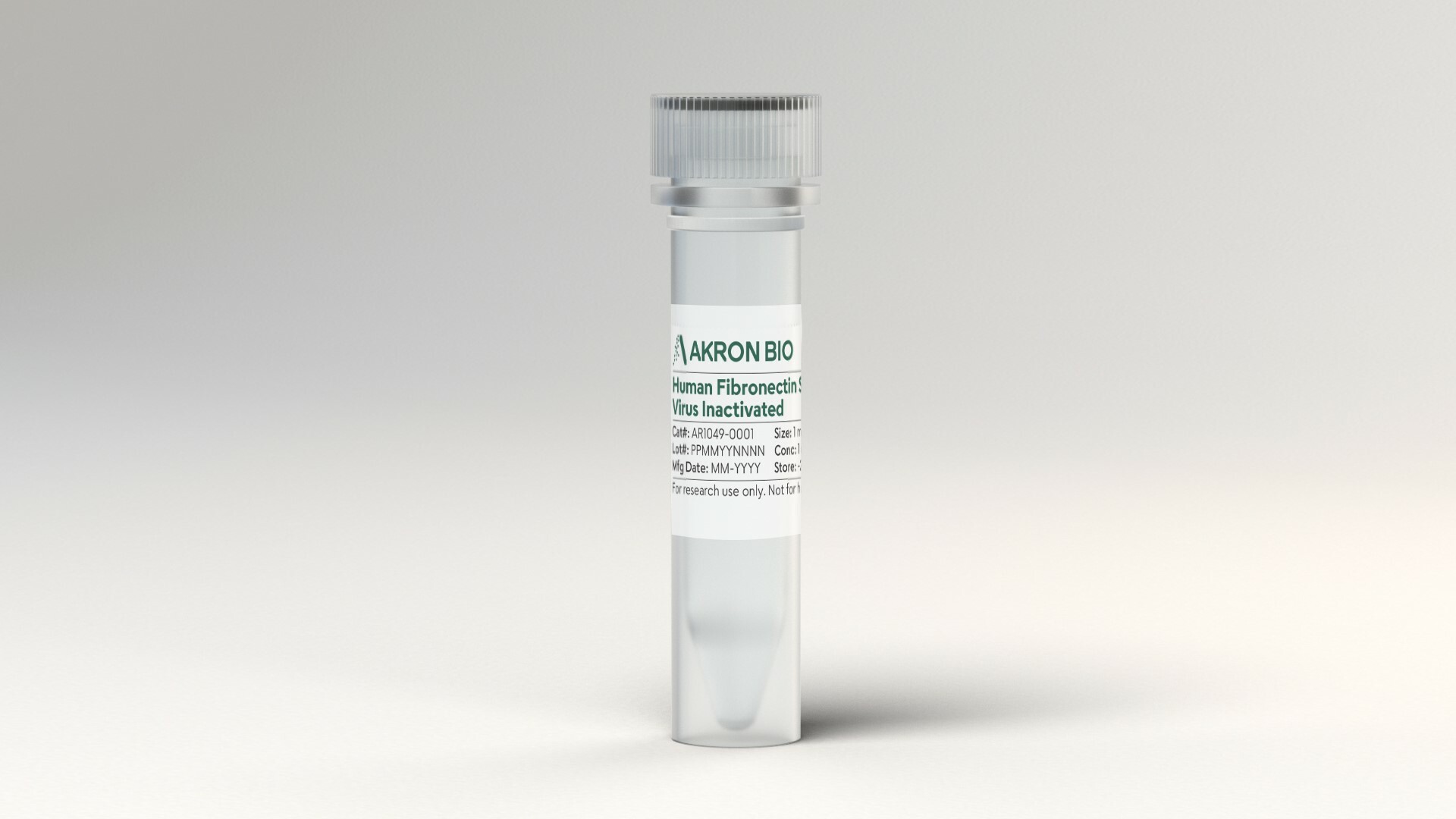
Human Fibronectin, Virus Inactivated, Research Grade
Custom Services
Custom size and configuration vials, syringes, bags and more. We manufacture custom recombinant proteins, media and cell and gene therapy products based on your specifications, including all under cGMP.
Capitalizing on our established platform for manufacturing cGMP-compliant clinical and commercial grade ancillary products. Our broad expertise and vision are highly sought after, particularly for challenging projects which demand carefully considered approaches.
