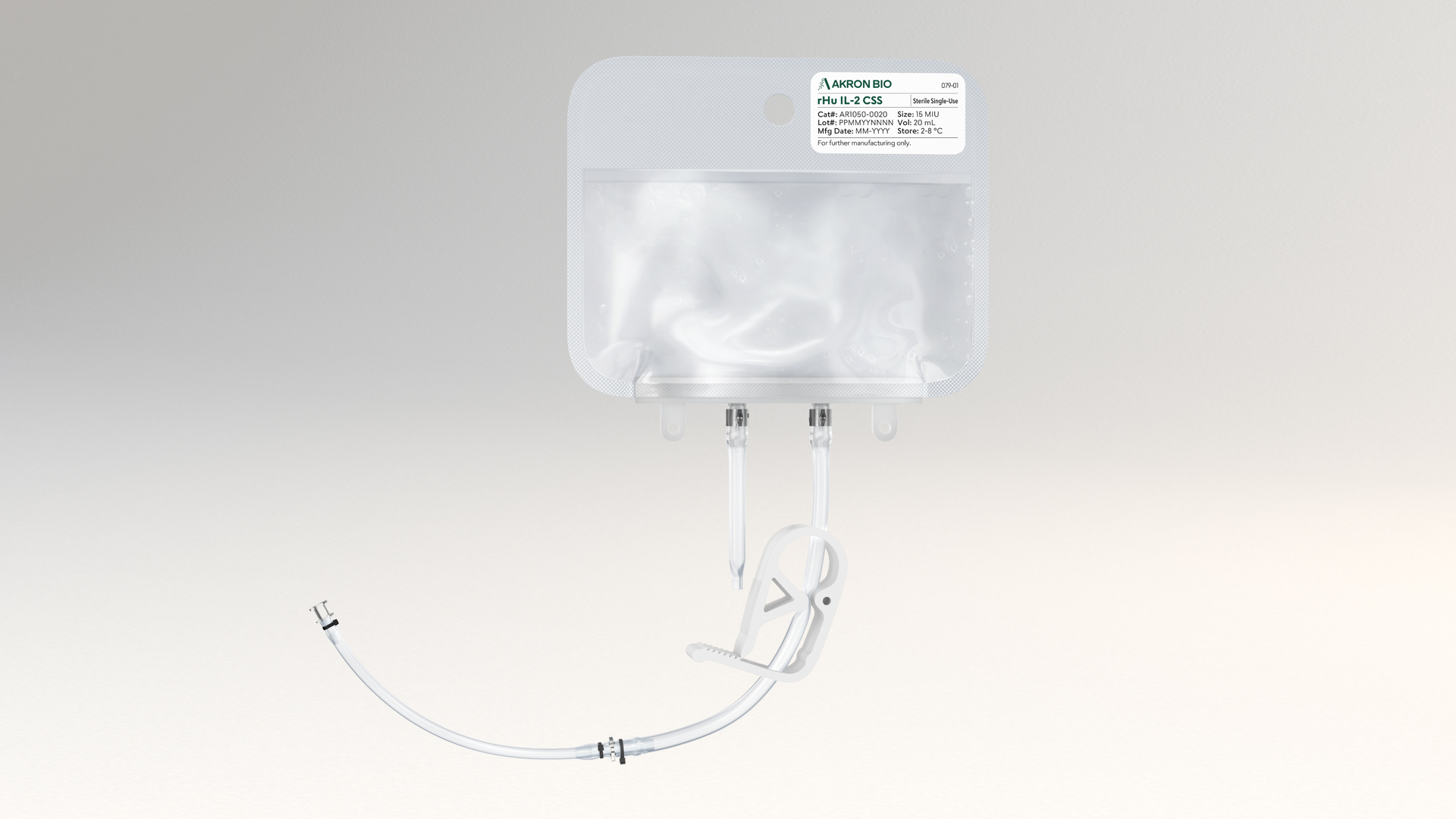
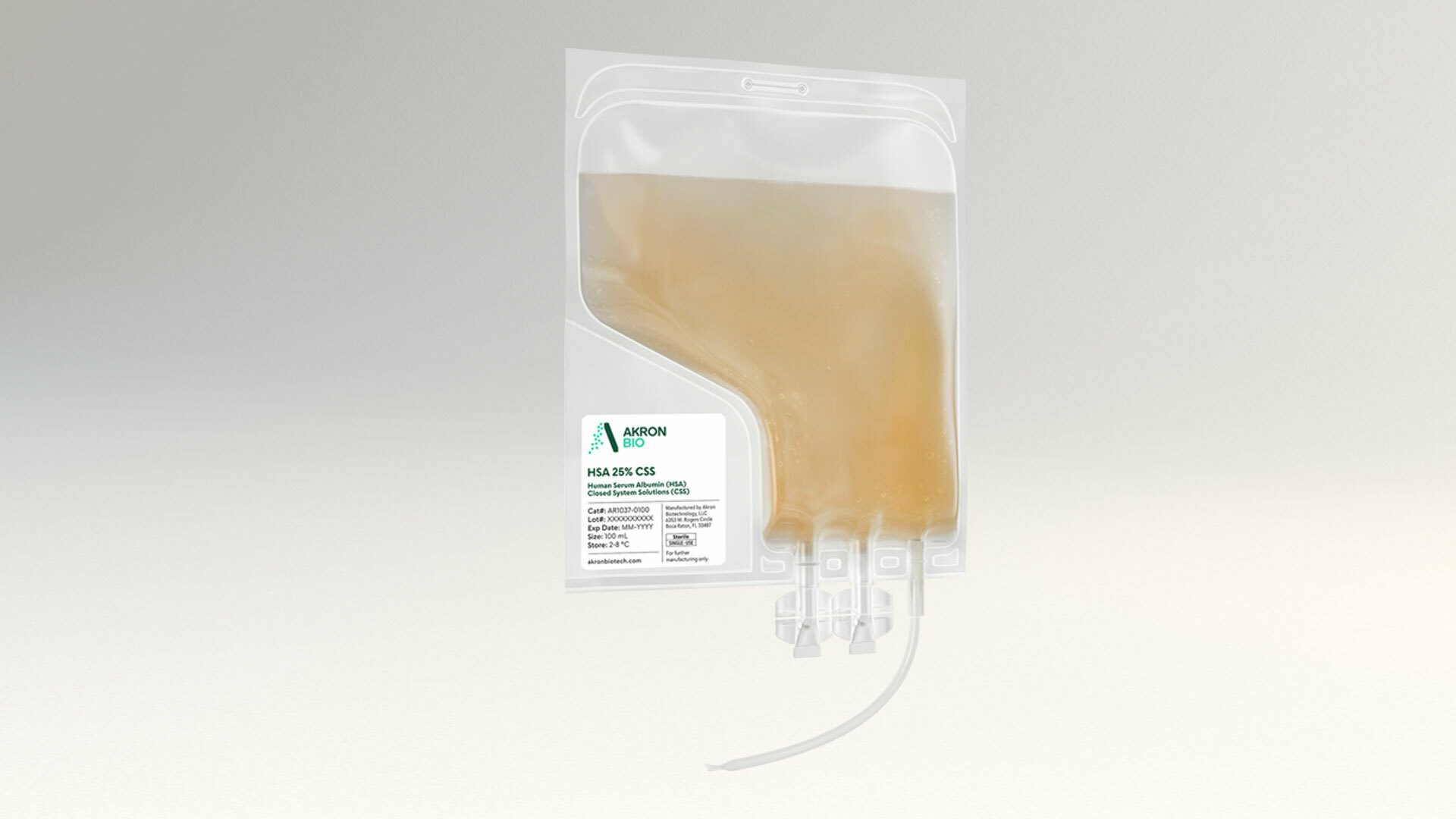
Closed System Solutions™ (CSS) line of cGMP Products
Akron’s CSS product line functionally closes critical unit operations in cell and gene therapy manufacturing workflows
CSS products are available in single-use, ready-to-use bags with weldable tubing to provide consistency and sterility assurance. Akron has engineered its CSS product line to integrate optimally dosed, cGMP-compliant blood-derived products and liquid cytokines across manufacturing platforms.
Akron’s CSS products leverage existing cGMP manufacturing processes and have Drug Master Files with FDA for seamless regulatory support. They are available in several volumes and concentrations with multiple connection options, enabling preclinical to commercial cell and gene therapy manufacturing.
Watch for more products in our CSS line available later in 2023.
COMING SOON
Custom Services
Custom size and configuration vials, syringes, bags and more. We manufacture custom recombinant proteins, media and cell and gene therapy products based on your specifications, including all under cGMP.
Capitalizing on our established platform for manufacturing cGMP-compliant clinical and commercial grade ancillary products. Our broad expertise and vision are highly sought after, particularly for challenging projects which demand carefully considered approaches.