cGMP rHu IL-2 Closed System Solutions™
rHu IL-2 outfitted for closed-system manufacturing
Akron’s Recombinant Human Interleukin-2 (rHu IL-2) Closed System Solutions™ (CSS) is manufactured following all relevant cGMP guidelines for ancillary materials. The rHu IL-2 active substance is supported by a Type II Master File (MF) on file with the FDA and an MF Type I on file with Health Canada, which can be referenced during your drug or biologic application process.
Our rHu IL-2 amino acid sequence is identical to Proleukin® (aldesleukin), and its functional similarity in T Cell expansion has been evaluated and confirmed. Akron’s rHu IL-2 is a single chain, 15.3 kDa, non-glycosylated lymphokine analog expressed in E. coli, containing 133 amino acids. The downstream purification process uses a multi-step orthogonal approach, without the use of affinity tags, to minimize exogenous impurities and ensure the delivery of highly purified and active substance for further manufacturing applications.
The liquid rHu IL-2 CSS product is packaged in a sterile fluoropolymer bag with two different weldable tubing connection options, allowing for easy incorporation into modern closed-system cell culture bioprocessing protocols. rHu IL-2 CSS increases safety and ease of use by eliminating the reconstitution step during manufacture and allows for the introduction of cytokine material into culture media in a fully contained manner to maximize sterility. Sterile filtration and aseptic filling are followed by Endotoxin and Sterility testing performed per USP/EP on the final product. See product Packaging features below.
Active Substance
• Amino acid sequence identical to Proleukin® / aldesleukin
• Type II eCTD MF (#026152) on file with FDA and MF Type I (#e250089) on file with Health Canada
• Carrier protein-free formulation
• All raw materials are compliant, controlled, and traceable under Akron’s Quality Management System (QMS)
Manufacturing
• Multi-step downstream purification strategy excluding affinity tags
• Commercial-scale production capacity
• Sterile filtration and aseptic filling
Packaging
• Sterile bag chamber – 8-mil fluoropolymer film providing inert bio-reactivity, structural integrity, and minimal gas transmission
• Two-part weldable tube - top 6” portion made from weldable TPE AdvantaFlex® (1/8” ID x 1/4” OD), and the bottom 6” portion made from standard weldable PVC (3/32” ID x 5/32” OD)
• Primary packaging is plasticizer free
• Primary packaging materials extensively validated, controlled, and qualified to ensure a consistent experience
• Every bag packed in individual protective cassette and shipped in validated CSafe Parcel insulated shipper
Quality
• Relevant cGMP guidelines used in manufacture, testing, and release
• USP <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
• EP 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products
• ISO 13485:2016, Medical Devices - Quality Management Systems - Requirements for Regulatory Purposes
• ISO/TS 20399-1-3:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products
Stability
• Under long-term stability program
• Store at 2-8 °C
• Transport with cold packs
• Do not freeze
Methods of Use
There are two different options available for connecting to and extracting the liquid solution from Akron’s rHu IL-2 CSS product (for full instructions, see “Methods of Use” document):
1) Weldable connection via top TPE AdvantaFlex® section (1/8” ID x 1/4” OD)
2) Weldable connection via bottom PVC section (3/32” ID x 5/32” OD)
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications only. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
• Appearance (Visual inspection)
• pH (potentiometric)
• Molecular Weight (reducing SDS-PAGE)
• Impurities (reducing SDS-PAGE)
• Impurities (non-reducing SDS-PAGE)
• Biological Activity (HT-2 cell proliferation)
• Bacterial Endotoxins (USP <85>/ EP 2.6.14)
• Sterility (USP <71>/ EP 2.6.1)
Akron rHu IL-2 vs Proleukin® - pSTAT5 Expression

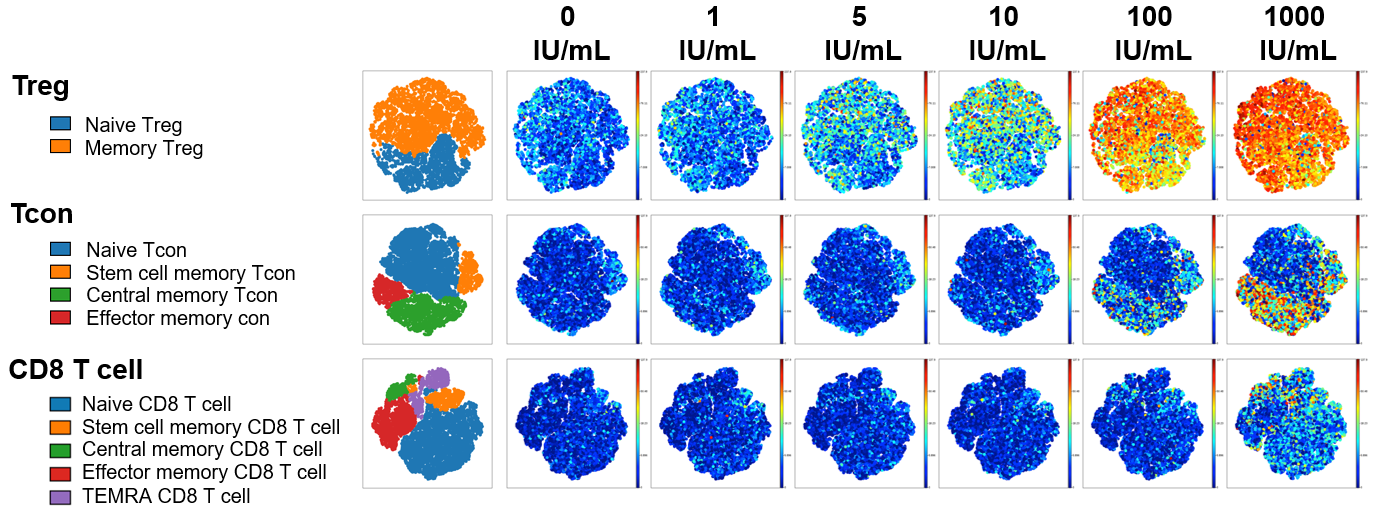
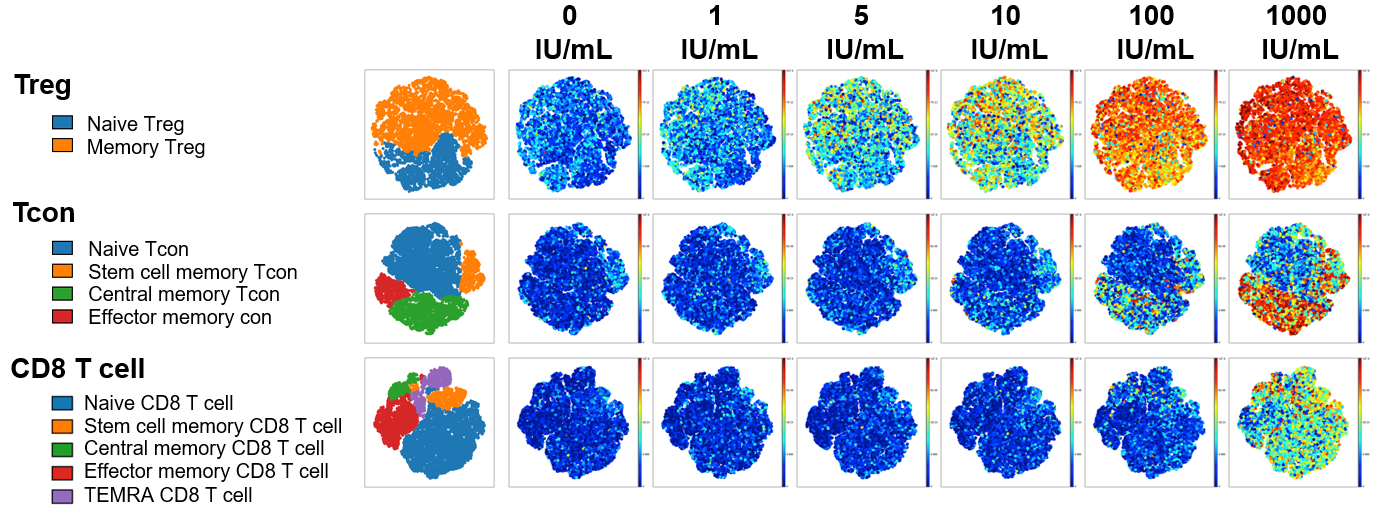
Figure 1: To examine IL-2 signaling, freshly isolated PBMC from healthy donors were stained with surface antibodies targeting 21 different protein markers prior to in vitro stimulation with IL-2. Single mass cell cytometry was used to compare the effect of Akron’s rHu IL-2 (top) against Proleukin® (bottom) on the expression of pSTAT5, pSTAT3, & pSTAT1 in T cell subsets. (pSTAT5 example shown above).
When results were summarized for six healthy donors, T cell stimulation in vitro by Proleukin® and Akron rHu IL-2 were indistinguishable.
This study was done in collaboration with the Dana-Farber Cancer Institute; study and poster available upon request.
1. Why use Akron’s Recombinant Human Interleukin-2 (rHu IL-2) Closed System Solutions (CSS)?
Akron’s novel rHu IL-2 CSS liquid formulation and sterile bag packaging increase safety and ease of use by eliminating the reconstitution step during manufacture, allowing for the direct introduction of cytokine material into culture media in a fully contained manner.
Akron’s Recombinant Human Interleukin-2 (rHu IL-2) is manufactured following all relevant cGMP guidelines for ancillary materials and is supported by a Type II Master File (MF) with the FDA and a MF Type 1 with Health Canada. It is purified in a pharmaceutical facility without the use of histidine tags and nickel columns. Sterile filtration with aseptic filling are followed by Endotoxin and Sterility testing performed per USP/EP on the final product. Our rHu IL-2 amino acid sequence is identical to Proleukin® (aldesleukin), and its functional similarity in T cell expansion has been evaluated and confirmed (see Product Page).
2. What are the recommended storage conditions for Akron’s rHu IL-2 CSS?
We recommend storing this product at 2-8 °C.
3. What is the white precipitate inside the bag that is observed while storing under recommended conditions?
During storage at 2-8 °C, a white precipitate can appear inside the bag as a result of a reversible interaction between the rHu IL-2 protein and the excipients at this temperature. The white precipitate disappears after a few minutes at room temperature and has no relevant impact on the strength, purity, or biological activity of Akron’s rHu IL-2. Once the precipitate has reached dissolution, a colorless, clear liquid should be observed within the bag, ensuring that it is ready to use.
Note: Do not use the bag until the precipitate completely dissolves and is no longer visible.
4. What are the shipping conditions for Akron’s rHu IL-2 CSS?
This product ships with cold packs. rHu IL-2 CSS units are packed in storage cassettes and shipped in validated insulated CSafe Parcel shippers.
5. What is the shelf-life for Akron’s rHu IL-2 CSS?
This product is currently under a long-term stability program.
6. What organism is used to express Akron’s rHu IL-2?
This product is expressed in E. coli.
7. How is Akron’s rHu IL-2 different than native IL-2?
Akron’s rHu IL-2 is not glycosylated because it is derived from E. coli and has an identical amino acid sequence to Proleukin® (aldesleukin), which differs from the native IL-2 amino sequence in the following ways: Akron’s rHu IL-2 does not have an N-terminal alanine, and it has a serine substituted in place of a cysteine at position 125. It is likely that the aggregation state is also different than native IL-2.
8. What is the molecular weight of Akron’s rHu IL-2?
Akron’s rHu IL-2 was characterized by liquid chromatography-electrospray ionization-tandem mass spectrometry analysis and was found to have an observed molecular mass of 15,319 Da. Every batch of product is released using a reducing SDS-PAGE molecular weight assay to directly compare against a reference standard.
9. Do you have a Master File (MF) available for this product?
The active substance in this product, Akron’s rHu IL-2, has a Type II eCTD MF (#026152) on file with the FDA and an MF Type I (#e250089) on file with Health Canada. We can provide you a Letter of Authorization that will permit these agencies to refer to the information on file in support of your submissions.
10. Is a TSE/BSE statement available for this product?
Yes, a TSE/BSE statement is available upon request.
11. Is virus and pathogen inactivation included in the manufacturing process?
No, because viruses that infect bacteria (bacteriophages) do not pose a known threat to human cells. Virus reduction manufacturing steps are usually not included when purifying material from a bacterial host, as is the case with Akron’s rHu IL-2.
12. What safety testing is done on this product?
Every lot of final product is tested with methods per USP/EP, for both Endotoxin (USP <85> / EP 2.6.14) and Sterility (USP <71> / EP 2.6.1).
13. Which cell types are suitable?
Akron’s cGMP-compliant rHu IL-2 can be used to promote the activation and proliferation of numerous immune cell types, including CAR-T cells, TCR-T cells, Tregs, TILs, NK cells, CIK cells, B cells, monocytes, and macrophages.
14. Do you have an SDS for this product?
Yes, an SDS is available upon request for this product.
15. How does Akron measure activity for rHu IL-2?
Akron uses a validated leukocyte proliferation assay to report activity. The rHu IL-2 induced proliferation of T lymphocyte HT-2 cells is measured against the World Health Organization (WHO) 2nd international standard for Interleukin-2, held by the National Institute for Biological Standards and Control (NIBSC 86/500). Akron uses a parallel-line concentration-response model to calculate a relative potency, per guidelines set forth in USP <1032> and USP <1034> (see Technical Overview).
16. Is the performance comparable to competitor rHu IL-2 products?
The specific activity (MIU/mg) of Akron’s rHu IL-2 batches have historically been comparable to the specific activity of the current international standard (NIBSC 86/500) for IL-2, known to be approximately 13.73 MIU/mg. Akron’s rHu IL-2 amino acid sequence is identical to Proleukin® (aldesleukin), and its functional similarity in T Cells has been evaluated and confirmed (see Product Page Data).
17. What is the intended use for the product?
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
18. What packaging options are available for rHu IL-2 CSS?
Akron’s rHu IL-2 CSS comes packaged in a sterile 25 mL fluoropolymer bag chamber. The outlet tube is made from two different weldable materials to choose from. The top portion is made from weldable TPE AdvantaFlex® (1/8” ID x 1/4” OD) and the bottom portion is made from standard weldable PVC (3/32” ID x 5/32” OD). These packaging materials are extensively validated, controlled, and qualified to ensure a consistent experience.
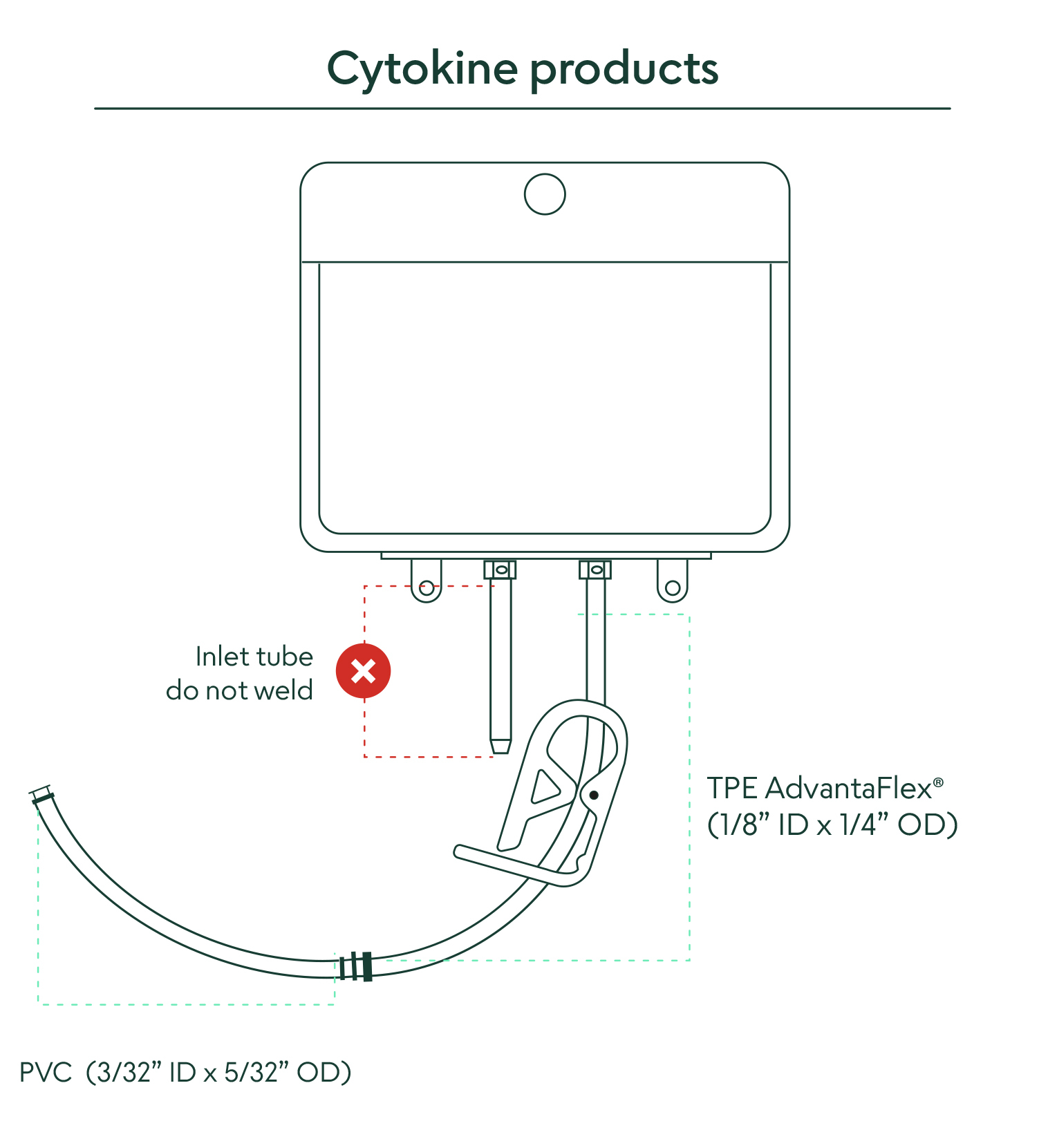
19. What closed-connection options are available to remove the material from the bag?
There are two different tubing materials available to make weldable connections to your closed-system process line (for full instructions, see “Methods of Use” document).
IL-2 plays a major role in both upregulating and downregulating the body’s immune response. It is critical for the homeostasis and differentiation of many immune cell types and is involved in the immune system’s ability for self-tolerance. The pleiotropic nature of cytokines is especially diverse in IL-2 due to its signal being transduced by at least three different primary signaling pathways. The trimeric IL-2 receptor protein (IL-2R) shares an identical subunit with the IL-7, IL-15, and IL-21 receptor proteins and activates some of the same signal transduction mechanisms. Akron’s cGMP-compliant rHu IL-2 can be used to promote the activation and proliferation of numerous immune cell types, including CAR-T cells, TCR-T cells, Tregs, TILs, NK cells, CIK cells, B cells, monocytes, and macrophages.
Aliquot Sizes & Formats
Bags (1 MIU) Cat. # AR1045-0010 (10 μg/mL)
Bags (2 MIU) Cat. # AR1045-0020 (10 μg/mL)
Bags (7.5 MIU) Cat. # AR1050-0010 (75 μg/mL)
Bags (15 MIU) Cat. # AR1050-0020 (75 μg/mL)