cGMP rHu IL-7 Lyophilized
Exceptional purity, consistency and performance. Expand T-Cells and NK-Cells with Akron’s cGMP IL-7.
Akron’s Recombinant Human Interleukin-7 (rHu IL-7) is manufactured following all relevant cGMP guidelines for ancillary materials and is supported by a Type II Master File (MF), which can be referenced by the FDA during your drug or biologic application process. Akron’s rHu IL-7 is a single chain, 17.5 kDa, non-glycosylated cytokine analog expressed in E. coli, containing 153 amino acids.
It is purified in a pharmaceutical facility without the use of histidine tags and nickel columns. Sterile filtration with aseptic filling and lyophilization are performed in-house with Endotoxin and Sterility testing conducted per USP/EP on the final product; the lyophilized product is packaged in sterile vials.
Active Substance
• Carrier protein-free formulation
• E. coli expression system
• All raw materials are compliant, controlled, and traceable under Akron’s Quality Management System (QMS)
Manufacturing
• Type II eCTD MF (#026084) on file with FDA
• Tag-free pharmaceutical processing
• Gram-scale production capacity
• In-house sterile filtration with aseptic filling and lyophilization
Quality
• Relevant cGMP guidelines used in manufacture, testing, and release
• USP <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
• EP 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products
• ISO 13485:2016, Medical Devices - Quality Management Systems - Requirements for Regulatory Purposes
• ISO/TS 20399-1-3:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products
• High Purity, Low Endotoxin - Endotoxin and Sterility testing per USP/EP
Stability
• 24-month shelf life
• Store at 2-8 °C
• Transport with cold packs
Reconstitution
Reconstitute with water for injection (WFI). Product is fully soluble in aqueous solution at 0.1 mg/mL.
For Use Statement
For research use or further manufacturing use in ex vivo cell therapy applications only. cGMP grade recombinant protein products are not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
• Appearance
• pH
• Residual Moisture
• Specific Activity
• Biological Activity
• Molecular Weight
• Western Blot
• Bacterial Endotoxins
• Sterility
1. Why use Akron’s Recombinant Human Interleukin-7 (rHu IL-7)?
Akron’s Recombinant Human Interleukin-7 (rHu IL-7) is manufactured following all relevant cGMP guidelines for ancillary materials and is supported by a Type II Master File (MF) with the FDA. It is purified in a pharmaceutical facility without the use of histidine tags and nickel columns. Sterile filtration with aseptic filling and lyophilization are performed in-house with Endotoxin and Sterility testing performed per USP/EP on the final product.
2. What are the recommended storage conditions for Akron’s rHu IL-7?
We recommend storing this product at 2-8 °C.
3. What are the shipping conditions for Akron’s rHu IL-7?
This product ships on cold packs.
4. What is the shelf-life for Akron’s rHu IL-7?
This product has a shelf life of 24 months after the date of manufacture under recommended controlled storage conditions.
5. Do you have any excursion stability data available?
No excursion data is currently available for this product, but please inquire about any specific stability needs you may have.
6. How do you reconstitute Akron’s rHu IL-7?
It is recommended that you reconstitute using water for injection (WFI). This product is fully soluble in aqueous solution at 0.1 mg/mL.
7. How long can I store Akron’s rHu IL-7 after reconstitution?
We do not suggest an ideal storage medium or storage conditions for reconstituted aliquots, as this product is intended to be used in a single application within cGMP manufacturing protocols. Storage after reconstitution is affected by many factors such as benchtop methodology, concentration, diluent, storage temperature, etc.
8. What organism is used to express Akron’s rHu IL-7?
This product is expressed in E. coli.
9. How is Akron’s rHu IL-7 different than native IL-7?
Akron’s rHu IL-7 is not glycosylated because it is derived from E. coli, and it contains an N-terminal methionine. It is likely that the aggregation state is also different than native IL-7.
10. What is the molecular weight of Akron’s rHu IL-7?
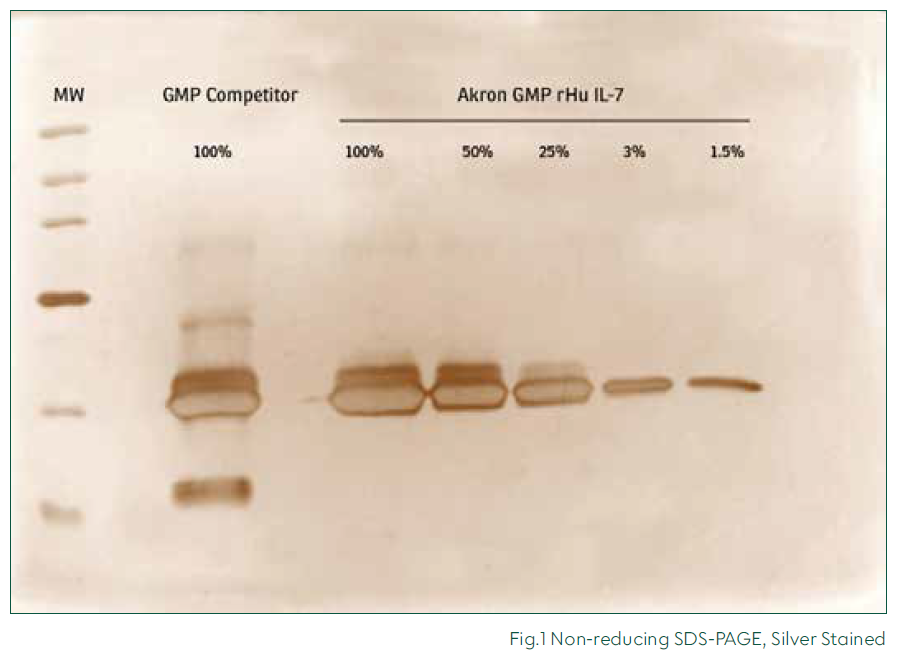
Akron’s rHu IL-7 has a theoretical molecular weight of 17,518 Da. Akron’s rHu IL-7 apparent molecular weight is indistinguishable when compared to a reference standard using Non-Reducing SDS-PAGE. Every batch of rHu IL-7 is analyzed by SDS-PAGE and must meet the molecular weight release specification between 15,500-18,500 Da.
11. Do you have a Master File (MF) available for this product?
Yes, our rHu IL-7 has a Type II eCTD MF (#026084) on file with FDA. We can provide you a Letter of Authorization that will permit the FDA to refer to the information on file in support of your submissions.
12. Does this product have a TSE/BSE statement?
Yes, a TSE/BSE statement is available upon request for this product.
13. Are animal-derived materials used in the manufacture of this product?
All raw materials, components, sub-components, and consumables used in the manufacturing of this product are either animal-free or in compliance with EMEA/410/01 rev. 3. Please see the BSE-TSE Statement.
14. Is virus and pathogen inactivation included in the manufacturing process?
No, because viruses that infect bacteria (bacteriophages) do not pose a known threat to human cells. Virus reduction manufacturing steps are usually not included when purifying material from a bacterial host, as is the case with Akron’s rHu IL-7. We use an E. coli based expression system that does not require additional virus reduction.
15. What safety testing is done on this product?
Every lot of final product is tested and released, with specifications and methods per USP/EP, for both Endotoxin (USP <85> / EP 2.6.14) and Sterility (USP <71> / EP 2.6.1).
16. Which cell types are suitable?
Akron’s cGMP-compliant rHu IL-7 can be used to stimulate proliferation of cytotoxic T lymphocytes (CTLs), B lymphocyte cells, monocytes, NK cells, and LAK cells.
17. Do you have an SDS for this product?
Yes, an SDS is available upon request for this product.
18. How does Akron measure activity for rHu IL-7?
Akron uses a validated leukocyte proliferation assay to report activity. The rHu IL-7 induced proliferation of 2E8 mouse B lymphocyte cells are measured against the proliferation caused by the World Health Organization reference standard for Interleukin-7 (NIBSC 90/530). Akron uses a parallel-line concentration-response model to calculate a relative potency, per guidelines set forth in USP <1032> and USP <1034>. (see Technical Overview)
19. Is the performance comparable to other rHu IL-7 formulations?
The specific activity (MIU/mg) of Akron’s rHu IL-7 batches have historically been comparable to the specific activity value of the World Health Organization standard (NIBSC 90/530) for IL-7, known to be
approximately 100 MIU/mg.
20. What is the intended use for the product?
For research use or further manufacturing use in ex vivo cell therapy applications. This product is not intended for direct in vivo use or for direct clinical use as a drug, therapeutic, biologic, or medical device.
21. What packaging options are available?
Akron’s lyophilized rHu IL-7 is packaged into generic colorless vial bottles made from Type I borosilicate glass that are sterilized before aseptic filling. These ampoule bottles have a nominal fill volume of 2.0 mL with a minimum overflow volume of 3.0 mL and have been shown to have an arsenic content of < 0.1 ppm. The bottles are closed with a gray bromobutyl rubber stopper and sealed with a flip-top aluminum seal that are both sterile before aseptic filling. Akron also offers our rHu IL-7 in a liquid formulation packaged into closed-system bags coming soon (inquire for details on bags).
22. Do I need to use a sterile needle and syringe to remove the product from the vial?
No, our vial bottle packaging was chosen for easy access with a laboratory pipette.
IL-7 is primarily expressed by non-marrow-derived stromal and epithelial cells and is required for early T cell development inside the thymus and for T cell homeostasis and regeneration in the periphery. It promotes the differentiation of hematopoietic stem cells (HSCs) down the lymphoid cell lineage and optimizes T cell dendritic cell interaction. The dimeric IL-7 receptor protein (IL-7R) shares an identical subunit with the IL-2, IL-15, and IL-21 receptor proteins and activates some of the same signal transduction mechanisms. IL-7 is currently being investigated as an immunotherapy agent for various malignancies as well as HIV infection. Akron’s cGMP-compliant rHu IL-7 can be used to stimulate proliferation of cytotoxic T lymphocytes (CTLs), B lymphocyte cells, monocytes, NK cells, and LAK cells.
Aliquot Sizes & Formats
Lyophilized (40 μg) Cat. # AK9842-0040