Comparable Advantages of IL-2 Ancillary Material Use Over Pharmaceutical Reagents within Cell Therapy Manufacturing
One of the principal challenges that cell therapy companies face as they advance in the clinic towards regulatory approval is the sourcing of critical cGMP ancillary materials, including high-quality and consistent cytokines and growth factors. These molecules are critical to stimulating the immune cells that form the basis of these companies’ innovative therapeutics. Of the myriad cytokines and growth factors critical in cell therapy manufacturing, there is one that clearly stands out in terms of its clinical and commercial impact: Recombinant Human Interleukin-2 (rHu IL-2).
rHu IL-2 is vital in the proliferation and activation of various T cells, NK cells, and other human immune cells. It is so vital a foundation of immunotherapy that one form of the molecule (aldesleukin) has been approved in multiple jurisdictions for decades as an injectable drug product under the trade name Proleukin®.
Common Approaches to Sourcing rHu IL-2
The significant influence and wide use of rHu IL-2 within cellular therapy manufacturing processes have resulted in drug manufacturers searching for the most dependable and vetted IL-2 molecule to incorporate into their development processes. One common approach is to source the approved pharmaceutical version of rHu IL-2. This gives the cell therapy developer peace of mind regarding safety and quality, though it presents its own challenges regarding ease of procurement, consistency of supply, format suitability for manufacturing, and cost.
Akron’s Approach to Manufacturing rHu IL-2
Akron is one of the few manufacturers of GMP rHu IL-2 that offer the true aldesleukin variant, which means that our IL-2 amino acid sequence is identical to Proleukin®. By employing similar production processes and analytical strategies, Akron has been able to bring to market the same degree of safety and quality expected of a pharmaceutically licensed product while solving issues around supply, utility, and cost.
Moreover, Akron manufactures its IL-2 large scale, allowing cell therapy developers to source large quantities of material from fewer lots, reducing variability in their processes. The one-on-one communications we foster with our clients allow us to schedule production runs that are tailored to the unique demand schedules of commercial partners, ensuring a reliable and consistent supply.
With our unique product formulation and packaging, Akron has opened the possibility for simple, fully closed-system manufacturing. Our prefilled liquid syringes and liquid bags with weldable tubing reduce both the logistical steps involved for the end user and the risks involved in formulating media to support manufacturing. See more information on our Closed System Solutions™ (CSS) line of products here.
Robust Testing Shows That Akron’s IL-2 is Functionally Similar to Proleukin®
Although our rHu IL-2 product comes without the pharmaceutical price tag and sourcing challenges, it’s backed by robust lot-specific release testing using accurate and validated methodologies not found elsewhere in the ancillary material market. See our discussion regarding rHu IL-2 activity testing here. Extensive direct comparability assays between Akron’s IL-2 and Proleukin® have been completed, both in terms of molecular characterization and functional comparability studies that look at T-cell expansion across several subsets. A summary of these results is presented below, and you can read more about the study here.
To ensure the smooth integration of Akron’s rHu IL-2 into the cell therapy developer’s bill of materials and regulatory filings, the product is backed by a type II eCTD Master File (MF) with the U.S. FDA and a type I MF with Health Canada. Our team works directly with all clients to provide thorough regulatory and technical support from project inception all the way through commercialization. Indeed, Akron’s IL-2 now supports dozens of late-stage clinical and commercial programs.
Summary of Molecular and Functional Characterization Comparison between Akron rHu IL-2 and Proleukin®
Molecular Characterization
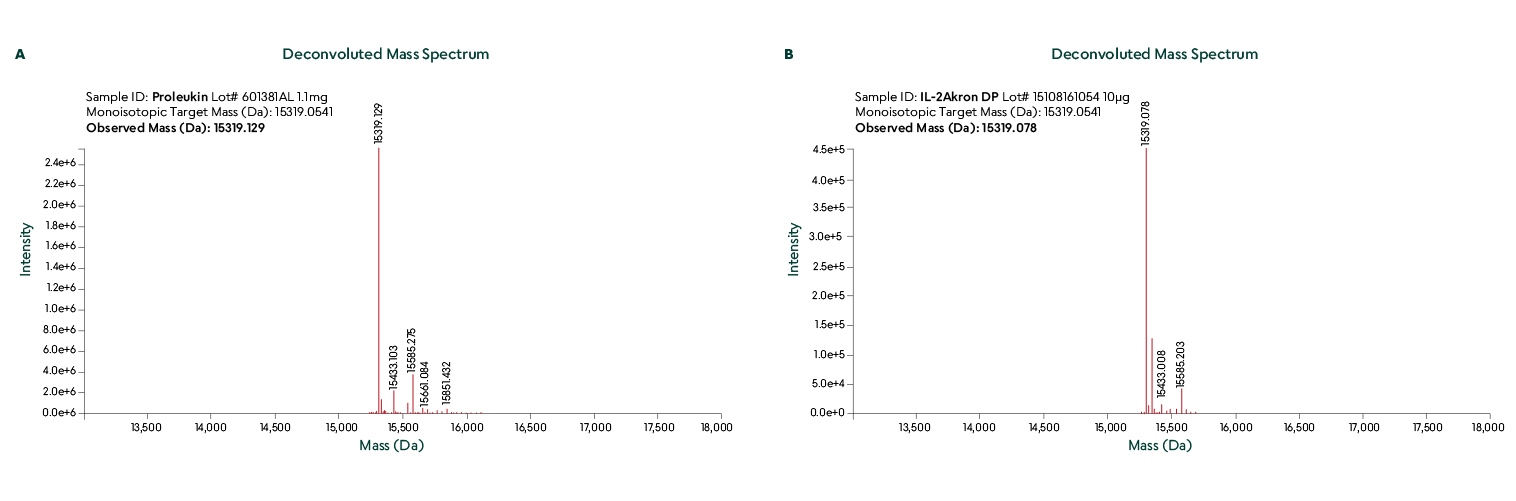
Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC-ESI-MS) was used to confirm identical primary structure and disulfide bridging.
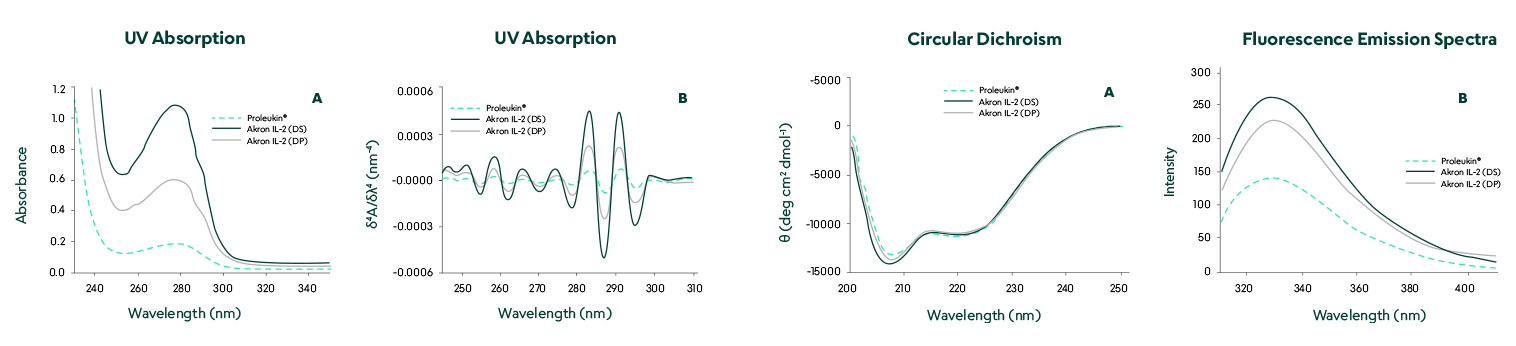
Structural comparison by UV absorption, circular dichroism, and fluorescence emission spectra showed no significant differences.
Gels from Reducing and Non-reducing SDS-PAGE visibly show Akron rHu IL-2 purity equal to, if not greater than, Proleukin®.
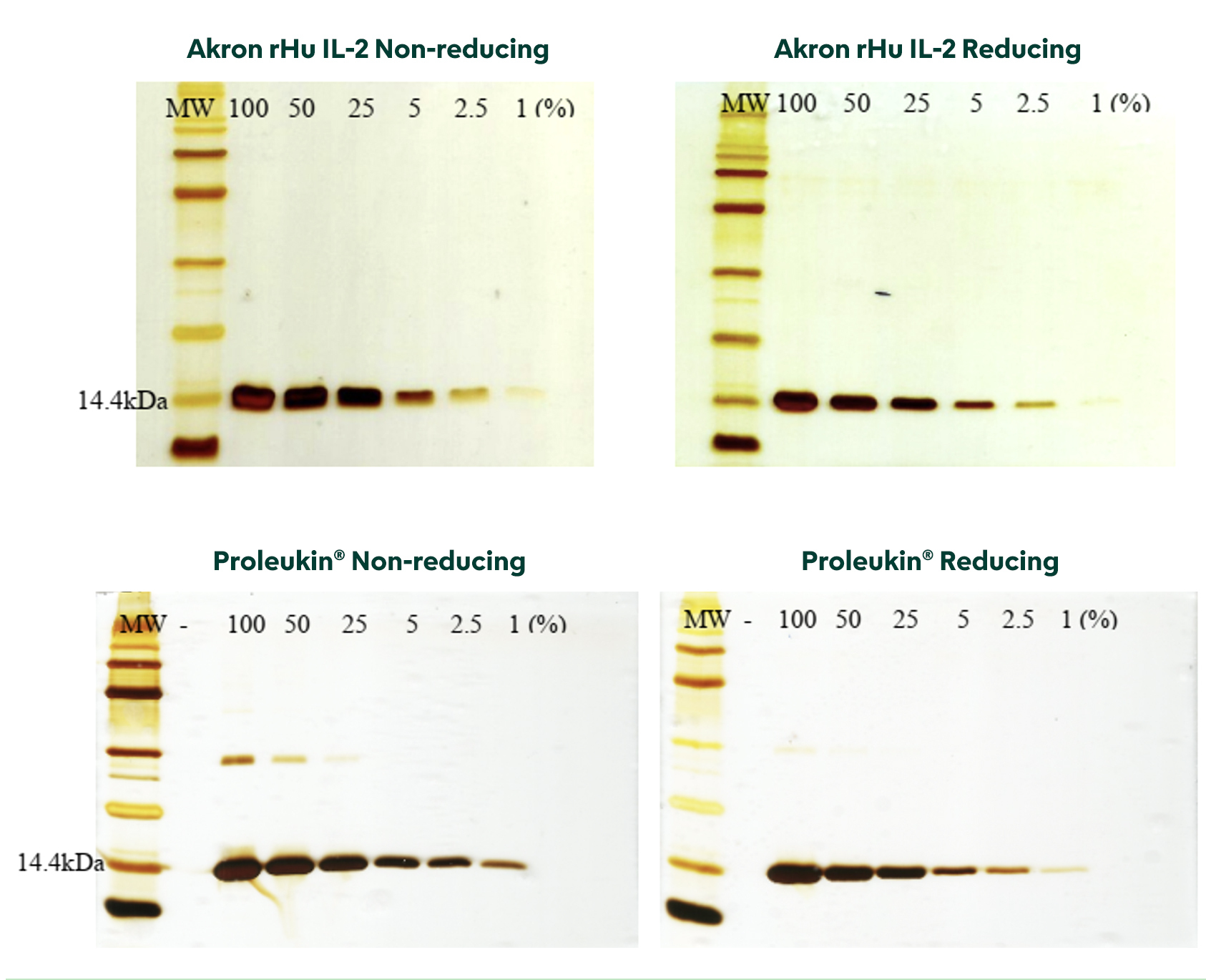
Functional Characterization
Freshly isolated PBMC from six healthy donors were stained with 21 different metal-labeled antibodies targeting different T cell-relevant protein structures. Single cell mass cytometry by time of flight (CyTOF) using an Inductively coupled plasma mass spectrometer (ICP-MS) was used to characterize cellular responses after in vitro stimulation with Akron’s rHu IL-2 and Proleukin®.
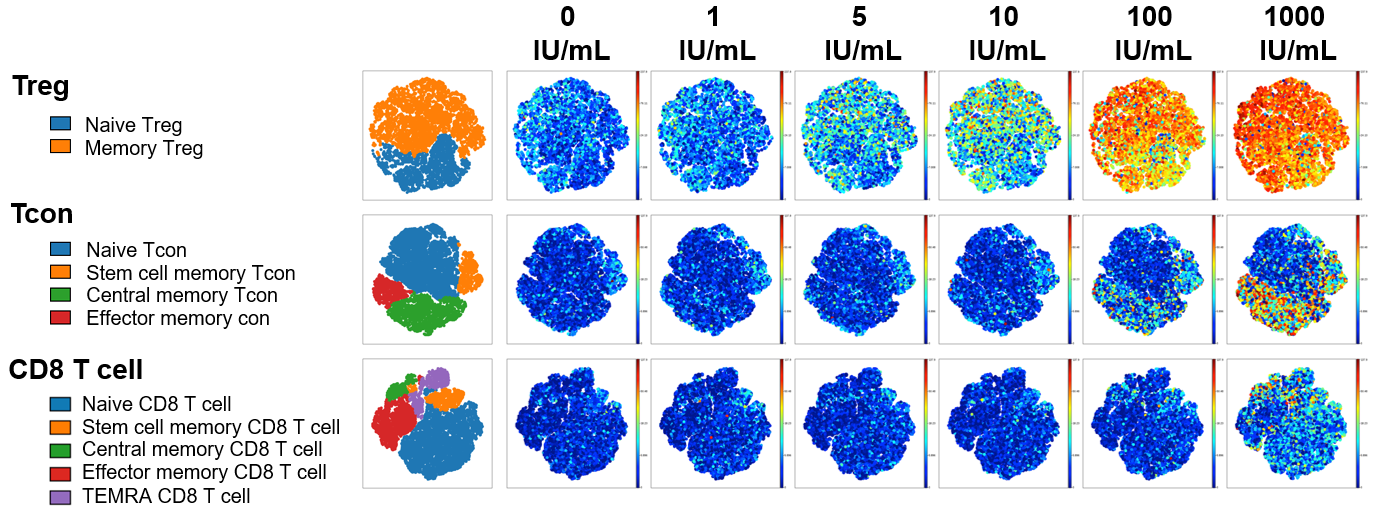
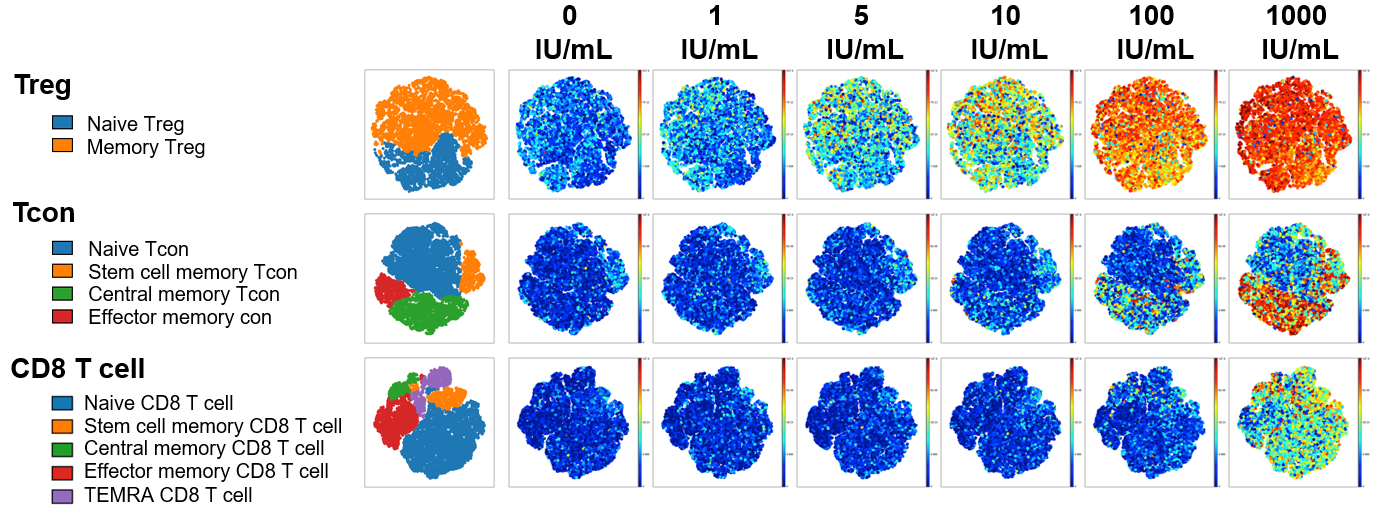
At low IL-2 concentrations (5-10 IU/ml), memory and naive Treg were selectively activated and only pSTAT5 was detected. At high rhIL-2 concentrations (100-1000 IU/ml), memory subsets of CD4Tcon and CD8 T cells were activated in addition to CD4Treg. In all T cell subsets at all ligand concentrations, IL-2 signaling was mediated primarily through pSTAT5 without significant activation of STAT1 or STAT3.
pSTAT5 expression example shown below visualized via t-distributed stochastic neighbor embedding (t-SNE). When results were summarized for all 6 healthy donors, T cell stimulation in vitro by Akron rHu IL-2 and Proleukin® were indistinguishable.
Akron rHu IL-2 vs Proleukin® - pSTAT5 Expression

The data above demonstrates the extreme similarity between Akron’s rHu IL-2 and Proleukin® in terms of molecular properties and T cell stimulation. The interchangeability between these two products gives cell therapy developers more options when sourcing high-quality and consistent reagents. The advantages of using a well-characterized and validated ancillary material compared to pharmaceutically licensed product for these processes becomes readily apparent when considering the scale of cell therapy commercialization and the advancements being made in closed-system manufacturing technologies. As the advanced therapy industry inevitably expands, resilient and mature reagent supply chains must rise to the call of meeting the stringent expectations of the final licensed therapies they support.
*Trademarks are property of their respective owners.